India derails trial to revive brain-dead accident victims amid controversy
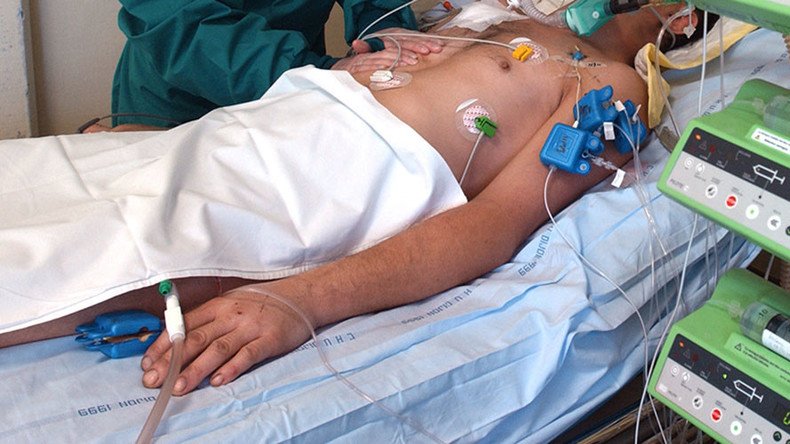
A controversial experiment which would have attempted to revive brain-dead accident victims in India has been scrapped by the country's medical research council. Those behind the trial have vowed to continue it outside India if necessary.
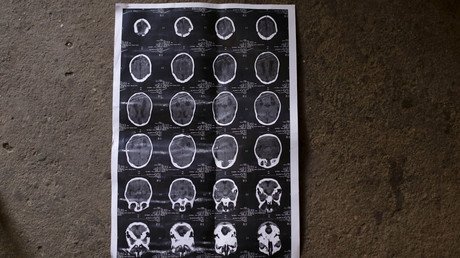
The Indian Council of Medical Research (ICMR), which deregistered the experiment from India's clinical trial registry on Friday, cited a number of problems with the ReAnima trial, which would have sought to bring brain-dead people back to a “minimal conscious state” in which patients show moments of consciousness, such as moving their eyes to follow objects.
Among the problems cited by the ICMR was failure to seek permission from the drug controller general of India, a requirement for all clinical trials in the country, Science reported.
But the man behind the trial, orthopedic surgeon Himanshu Bansal, says that ICMR has “nothing to do with the trial,” stressing that the matter is in the hands of the drug controller.
Meanwhile, Bioquark – a Philadelphia-based biotech firm that had agreed to supply the trial with peptides that are said to help regenerate brain cells – has said it is prepared to pursue the experiment outside of India if necessary.
“We are in no major rush...many road blocks, no doubt, will pop up. But the project will go on,” Bioquark chief executive officer Ira Pastor said.
The experiment, announced in May, seeks to give around 20 brain-dead people a mix of interventions including injections of mesenchymal stem cells and peptides, along with transcranial laser stimulation – the process of shining pulses of near-infrared light into the brain – and median nerve stimulation, the electrical stimulation of a major nerve that runs from the neck to the arm.
Both transcranial laser stimulation and median nerve stimulation have been shown to improve cognition in patients with traumatic brain injury.
In addition to the ICMR, a number of figures in the medical community have been critical of the experiment, with many expressing doubts that it would succeed. Bansal, however, insists that medical literature exists which describes a number of cases of people who have recovered to full consciousness from a minimal conscious state.
But Bansal did acknowledge during a June interview with The Wire that there is a possibility that patients could enter into a minimally conscious state but fail to progress further. Although he said his team had “not planned” for that scenario initially, he has since secured an insurance policy to cover the costs of full-time care for such patients.
Some have argued whether the trial is ethically sound, with some expressing concern that the method has not been tested on animal models. In response, Bansal has argued that there are no good animal models for the subject of human brain death.
But it isn't just medical professionals questioning Bansal's ambitious plan. Pastor admitted that the team has struggled to convince family members of brain-dead accident victims to enroll their loved ones in the trial.