First malaria vaccine approved after decades of research
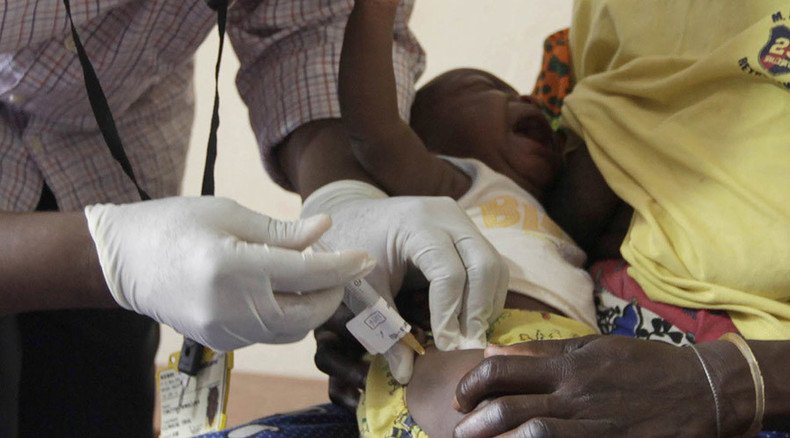
The world’s first malaria vaccine has been approved by the European Medicines Agency, the company that developed the new jab has announced.
It took more than 30 years for scientists to develop Mosquirix, also known as “RTS,S”. The vaccine was designed to prevent malaria in children aged from 6 weeks to 17 months in sub-Saharan Africa, which faces deaths of more than half a million children annually.
The World Health Organization will now assess how the vaccine might be used alongside other tools and give its recommendations on its implementation within African immunization programs once it is approved by national regulatory authorities, GSK pharmaceutical company said in a press release.
READ MORE: Alzheimer’s breakthrough? First ever drug found that may slow disease
The company says that it is committed to a not-for-profit price for the vaccine.
While other vaccines fight viruses or bacteria, RTS,S has been developed to tackle Plasmodium falciparum, the malaria parasites that affect people. It is found globally but most commonly in sub-Saharan Africa. The vaccine is said to help the immune system defend against the infection that enters the blood streams after a mosquito bite.
The company’s research was backed by the PATH Malaria Vaccine Initiative and the Bill & Melinda Gates Foundation. GSK began research on a malaria vaccine 30 years ago and started first vaccine trials in Africa in 1998 through research centers in Burkina Faso, Gabon, Ghana, Kenya, Malawi, Mozambique, Nigeria, and Tanzania. More than 16,000 children were involved in the trials.
The vaccination showed mixed results as the jab performed differently. After three doses of the vaccine, one month apart, with an additional fourth dose given 18 months later, malaria cases were reduced by almost half in children from the second group at the time of first vaccination and by one third in infants aged 6-12 weeks.
“Today marks a significant scientific milestone for the long-standing partnership to develop a vaccine, yet several more steps remain before a malaria vaccine might reach the young children in Africa who most need protection against this deadly human parasite,” said Dr. David C. Kaslow, Vice President of Product Development at PATH.
READ MORE: Marijuana may help heal broken bones – new study
Scientists say that malaria is a very hard disease to fight. Even though the vaccine itself did not perform as effective as the researchers had hoped, it can be still of a great help when used in addition to other malaria control measures such as bed nets.
"While RTS,S on its own is not the complete answer to malaria, its use alongside those interventions currently available such as bed nets and insecticides, would provide a very meaningful contribution to controlling the impact of malaria on children in those African communities that need it the most," said Andrew Witty, CEO of GSK, as cited by the Telegraph.
The vaccine will not be licensed for travelers, as it was specially developed for African children.












