Festive Champhysics: Uncorking sparkling wine a key to solving energy problems

When you were opening a bottle of champagne to mark the New Year, you probably had no idea that you triggered a complex physical process. Understanding it can lead to more efficient energy production, but requires a supercomputer.
The bubbles that give sparkling wine its texture and sometimes cause the liquid to spray out of the bottle are carbon dioxide, which remains dissolved under pressure but reveals itself after the cork pops.
The basics of the process are well known, but the exact laws governing how bubbles emerge and merge together are not yet fully understood, partially because they grow from nanometer and micron sizes to those measured in millimeters and centimeters.
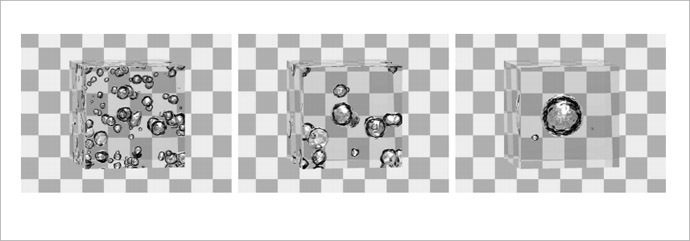
This evolution of bubbles is scientifically called Ostwald ripening after the researcher Wilhelm Ostwald, who discovered it in 1896. Basically, small bubbles are eaten by bigger ones because the bigger bubbles are in a more stable energy state.
Laymen usually see this phenomenon in food and beverages: be it champagne bottles at New Year’s, soda drinks or even ice cream, where the formation of ice crystals from a melted mixture is governed by the same principles.
But Ostwald ripening is also encountered in industrial equipment. Many power stations use boilers to convert water to steam to drive the turbines, and bubbles forming on surfaces decrease their efficiency by preventing heat exchange and slowly damaging the parts. They are also the scourge of shipbuilders, as they gradually cause deterioration in rotating propellers.

Understanding exactly how Ostwald ripening works can allow designing better boilers and turbines, and that is what a team of scientists from the University of Tokyo, Kyusyu University and RIKEN is doing. Their work was described in a report published by the Journal of Chemical Physics.
The researchers conducted the experiment not with a bottle of champagne, but with virtual molecules modeled by RIKEN's K supercomputer, the fastest in Japan and the fourth-fastest in the world. The computer used about 4,000 processors to help the team to simulate about 700 million particles and follow them through a million time steps.
"A huge number of molecules, however, are necessary to simulate bubbles – in the order of 10,000 are required to express a bubble,"said researcher Hiroshi Watanabe, one of the authors of the report.
"So we needed at least this many to investigate hundreds of millions of molecules – a feat not possible on a single computer."
The scientists confirmed that the Lifshitz-Slyozov-Wagner (LSW) theory, a classical 1960s theory explaining how bubbles form in foams, describes the Oswald ripening correctly, despite some evidence to the contrary.
“Honestly speaking, our present study is quite fundamental and it cannot be applicable to improve the efficiency of real devices right now,” Watanabe told Motherboard, an online magazine and video channel. “But this is the first step to understand how bubbles appear and how bubbles interact each other during the bubble formation from the molecular level.”
So the next time you open a bottle of champagne or soda, you can appreciate Oswald ripening in action and meditate on how gaining an insight into it could help solve the global energy crisis.












